The advent of next-generation sequencing (NGS) has allowed for the systematic investigation of the genomic and molecular alterations in EOC which can identify patients as candidates for individualized targeted therapy.

Once recurrent, ovarian cancer generally does not exhibit the same level of chemo-sensitivity, highlighting the need for rational therapies directed toward specific molecular targets. Standard therapy includes surgical debulking and platinum and taxane-based chemotherapy, resulting in complete clinical remission in up to 75% of patients, however only 30% of patients will be cured. The majority of women with epithelial ovarian cancer (EOC) are diagnosed with advanced disease. Ovarian cancer is the fifth leading cause of death in women in the United States with an estimated 22,280 new cases and 14,240 deaths in 2016. This strategy allows for rapid access to genomic information that can guide targeted treatment decisions and reduce the burden on genetic counselors, an often limited resource, who will only see patients with a positive somatic triage test. We will explore a proposed testing strategy using somatic tumor testing as an initial triage whereby those patients found with somatic testing to have HRD gene mutations are referred to genetics to determine if the mutation is germline. For now, germline and somatic tumor testing provide important and non-overlapping clinical information. The objective of this review article is to focus on the current germline and somatic contributors to ovarian cancer and the state of both germline and somatic HRD testing. In the near future, patients with germline or somatic HRD will likely be candidates for a growing list of targeted therapies in addition to poly (ADP-ribose) polymerase (PARP) inhibitors, and, as a result, establishing an infrastructure for widespread HRD testing is imperative. Furthermore, an additional 5–7% of ovarian cancer cases will have somatic HRD. The list of inherited mutations associated with ovarian cancer continues to grow with the literature currently suggesting that up to one in four cases will have germline mutations, the majority of which result in HRD.
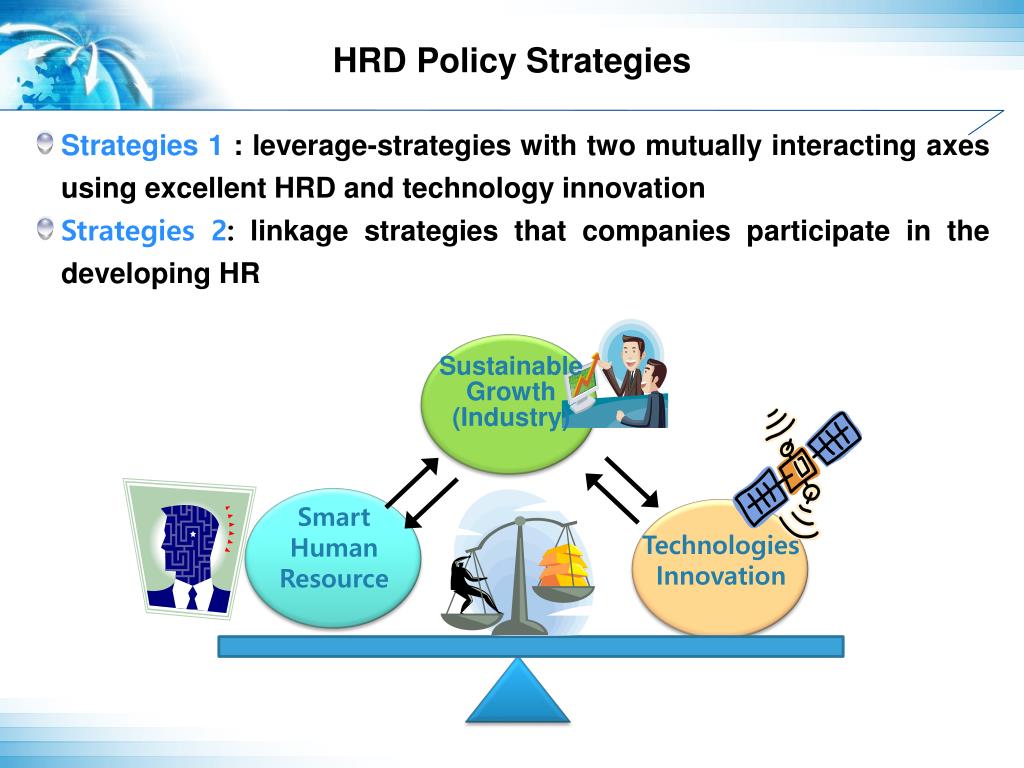

HRD impairs normal DNA damage repair which results in loss or duplication of chromosomal regions, termed genomic loss of heterozygosity (LOH). However, through germline and tumor sequencing an understanding of the larger phenomenon of homologous recombination deficiency (HRD) has emerged. Until recently our knowledge of a genetic contribution to ovarian cancer focused almost exclusively on mutations in the BRCA1/2 genes.


 0 kommentar(er)
0 kommentar(er)
